Transcriptomics of Adherent Endothelial Cells for Improved Endothelialization of Small-diameter Vascular Grafts
Coronary Artery Disease, or CAD, is the most common type of cardiovascular disease. Claiming nearly 400,000 lives annually, it is also the deadliest. Coronary Artery Bypass Grafting, also known as CABG, is a major surgical procedure that involves bypassing blockages in the coronary arteries to restore blood flow to the myocardium of the heart. The current state of CAD treatment still relies heavily on this well-established surgery. During surgical procedures, venous or arterial conduits—referred to as autografts—are harvested from the patient’s own body. For CABG, autografts are considered the “gold standard” graft because of their better long-term outcomes compared to the alternative synthetic, small-diameter grafts. However, they do come with several limitations and complications, such as limited availability and invasive harvesting of vessels, which can lead to additional complications and a higher risk of issues like graft failure and poor wound healing.
Developing a small-diameter vascular graft with a stable endothelial cell (EC) layer is critical for creating a vascular graft that remains patent and would help eliminate current graft issues such as thrombosis and neointimal hyperplasia. The goal of this project is to determine a method to create a stable EC monolayer on the inner surface of a vascular graft. The project focuses on studying the role of gene expression in endothelial cell adhesion using RNA sequencing and developing a novel method to strengthen endothelial cell retention on vascular grafts by enhancing the adhesion strength of the cells themselves.
 We have identified several downregulated genes in adherent endothelial cells, one of which was Fibronectin leucine-rich transmembrate protein 2 (FLRT2). We currently are using RNA-silencing approaches to transiently downregulate FLRT2 in human aortic endothelial cells (HAECs) to improve their adhesive properties. Once silences, we will seed them onto commercially available vascular grafts to check their behavior under fluid shear stress conditions to assess cell retention. In addition, we also look for changes in cell phenotype by looking for endothelial cell activation and dysfunction.
We have identified several downregulated genes in adherent endothelial cells, one of which was Fibronectin leucine-rich transmembrate protein 2 (FLRT2). We currently are using RNA-silencing approaches to transiently downregulate FLRT2 in human aortic endothelial cells (HAECs) to improve their adhesive properties. Once silences, we will seed them onto commercially available vascular grafts to check their behavior under fluid shear stress conditions to assess cell retention. In addition, we also look for changes in cell phenotype by looking for endothelial cell activation and dysfunction.
The outcomes could significantly advance the development of more effective vascular grafts, offering new treatment avenues for patients with limited options for recovery. By improving vascular tissue regeneration through innovative molecular and biomaterial strategies, this project aims to contribute to the broader goal of reducing the burden of cardiovascular disease and enhancing patient outcomes.
Image Description
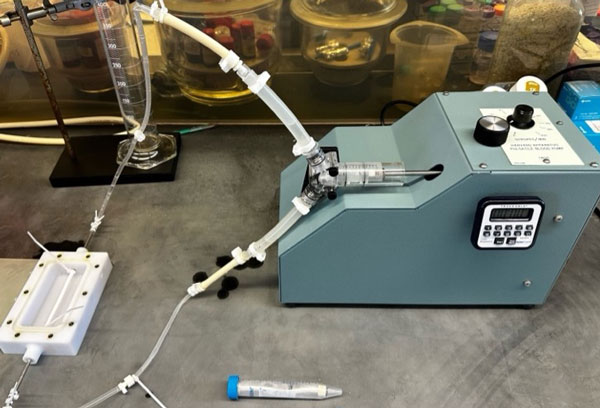
Setup for exposing cell-seeded grafts to pulsatile shear stress to investigate cell retention in a clinical small-vascular graft mimicking physiological conditions.
Related Publications
- Wolfe JT, Chen V, Chen Y, Tefft BJ. Identification of subpopulation of highly adherent endothelial cells for seeding synthetic vascular grafts. J Thorac Cardiovasc Surg. 2024 Jul 05 PMID: 38972570 PMCID: PMC11700231. SCOPUS ID: 2-s2.0-85200217289 07/08/2024.
- Shradhanjali A, Uthamaraj S, Dragomir-Daescu D, Gulati R, Sandhu GS, Tefft BJ. Characterization of Blood Outgrowth Endothelial Cells (BOEC) from Porcine Peripheral Blood. J Vis Exp. 2022 Jan 06(179) PMID: 35068481. PMCID PMC9645770. SCOPUS ID: 2-s2.0-85123744701 01/25/2022
- Wolfe JT, Shradhanjali A, Tefft BJ. Strategies for Improving Endothelial Cell Adhesion to Blood-Contacting Medical Devices. Tissue Eng Part B Rev. 2022 Oct;28(5):1067-1092 PMID: 34693761 SCOPUS ID: 2-s2.0-85140415060 10/26/2021
Project Leads
 Shashanka Rao, PhD
Shashanka Rao, PhD
Postdoctoral Researcher
Research Interests: cardiovascular tissue engineering, redox biology in the context of cardiovascular diseases such as hypertension and atherosclerosis
 Aysan Nazari
Aysan Nazari
Graduate Student
View more CaRE Lab Research

 We have identified several downregulated genes in adherent endothelial cells, one of which was Fibronectin leucine-rich transmembrate protein 2 (FLRT2). We currently are using RNA-silencing approaches to transiently downregulate FLRT2 in human aortic endothelial cells (HAECs) to improve their adhesive properties. Once silences, we will seed them onto commercially available vascular grafts to check their behavior under fluid shear stress conditions to assess cell retention. In addition, we also look for changes in cell phenotype by looking for endothelial cell activation and dysfunction.
We have identified several downregulated genes in adherent endothelial cells, one of which was Fibronectin leucine-rich transmembrate protein 2 (FLRT2). We currently are using RNA-silencing approaches to transiently downregulate FLRT2 in human aortic endothelial cells (HAECs) to improve their adhesive properties. Once silences, we will seed them onto commercially available vascular grafts to check their behavior under fluid shear stress conditions to assess cell retention. In addition, we also look for changes in cell phenotype by looking for endothelial cell activation and dysfunction.  Shashanka Rao, PhD
Shashanka Rao, PhD Aysan Nazari
Aysan Nazari
