A Ten-Year Milestone on the Road to Innovation
March 15, 2021
Science is a collaborative enterprise, and the journey an idea takes from inception to point-of-care is long and often winding. It may span years and pass from researcher to researcher as one investigator’s discovery fuels the next iteration of another’s innovation. Dr. Yu’s journey to develop medical solutions that positively impact the health of women, particularly those in low- and middle-income settings, tells a similar tale. The journey has spanned multiple institutions, iterations, and teams of collaborators. Now, at the beginning of its 10th year, Dr. Yu’s intraoperative breast tumor margin assessment project has gained some much-deserved attention, with recent innovations selected to grace the cover of the December 2020 issue of the Journal of Biomedical Optics.
Dr. Yu’s intraoperative breast tumor margin assessment project started with a problem: Lumpectomy, or breast conserving surgery (BCS), is performed in a half to two-thirds of all breast cancer cases. Some individuals who undergo BCS require a second surgery, as contemporary methods of analyzing the effectiveness of the preliminary surgery, which include ensuring that tumor margins are free of cancerous cells, often do not occur intraoperatively. And while intraoperative technologies do exist, further innovation is needed to ensure that intraoperative assessment is more accurate, faster, potentially easier to use, and more cost-effective.
In 2007, while completing his postdoctoral research at Duke University, Dr. Yu was asked by his adviser, Dr. Nirmala Ramanujam, to join a team working to develop a technology that would solve all of these problems. More specifically, the team at Duke was looking to develop a technique that could assess breast tissue tumor margins in real time. At that time, intraoperative margin assessment was accomplished with the use of a multiplexed device with 2x4 diffuse reflectance spectroscopy (DRS) probes, allowing for the non-invasive evaluation of tissue hemoglobin concentrations, oxygen saturation, beta-carotene concentration and scattering coefficient at eight tissue sites. The probe allowed for reasonably accurate differentiation between cancerous and noncancerous margins with a sensitivity of 74% and specificity of 86% in 70 lumpectomy specimens from 70 patients. However, this technology was large and difficult to maneuver, and because it would need to be repositioned many times during the assessment of each margin, a process that was time-consuming and cumbersome, this technology was eventually proven untenable.
In an attempt to reduce the amount of time spent repositioning assessment devices, Dr. Yu set out to increase the probe’s assessment area even further. With support of an NIH STTR Phase II grant awarded to Zenalux Biomedical, Inc.—a medical device company located in North Carolina and co-founded by Dr. Yu—Dr. Yu devised a seven-by-seven-channel probe that could effectively analyze an entire margin per positioning. By reducing the number of times the probe needed to be repositioned, the iteration decreased intraoperative assessment time to near-acceptable levels. However, the probe required direct contact with the specimen, increasing time spent in sanitization. The probe’s detectors had to be spaced over 5 mm apart from one another in order to reduce crosstalk, leaving small areas of the tumor margin unassessed, an unacceptable compromise in the quest to prove patients cancer free. In addition, the equipment was expensive, making the technology potentially inaccessible for large portions of the population.
In their next attempt, the team at Duke received a NIH BRP grant to build and test a photo-detector array that could be mass produced for the same purpose; however, the individual detectors composing the array still required direct contact with the specimen and still had to be spaced apart from one another. For those reasons, Dr. Yu concluded that DRS and other spectroscopy techniques proved a less-than-ideal solution for intraoperative assessment of breast tumor margins during BCS.
In 2012, Dr. Yu left Duke University for a position at the University of Akron, and while he did not complete any work related to breast tumor margin imaging during that time, the project was never far from his mind. Upon accepting his current position at Marquette University and the Medical College of Wisconsin (MU-MCW) in 2017, he was hungry for new ideas, eager to resume his work.
In January 2012, Dr. Anna Yaroslavsky’s group from the University of Massachusetts—Lowell, published new findings on the use of the contrast agent methylene blue (MB) and widefield fluorescence polarization imaging to distinguish between cancerous and non-cancerous cells. According to Yaroslavsky, “fluorescence polarization imaging identified the location, size, and shape of the tumor in all cases investigated,” and Yaroslavsky was able to use these findings, along with the confocal microscope, to produce high-contrast images with accurate quantification and without damaging any tissues, which meant that assessment using her method could be performed on fresh ex vivo breast lumpectomy specimens. Dr. Yu saw a great deal of merit in this application, but he also recognized some limitations. Due to the expense and limited field of view of the confocal microscope, Dr. Yu felt the approach could be improved upon. He would investigate the use of methylene blue as a contrast agent for differentiating between cancerous and non-cancerous cells, but he would look for ways to simplify the device by using the wide-field fluorescence imaging only.
In order to begin experimentation, Dr. Yu needed equipment, collaborators, and money. He discussed the project with Dr. Tina Yen, a breast cancer specialist from MCW’s Department of Surgery, and Dr. Taly Gilat-Schmidt, an imaging specialist from Marquette University, whose expertise would be instrumental to investigations regarding image stitching and contrast enhancement. They submitted a proposal to the WeCare Fund, a project managed by the Department of Surgery at MCW. WeCare is dedicated to providing seed money for projects that promise to further the fight for better patient care, and Dr. Yu’s project landed squarely within these criteria. As such, the proposal was accepted and the awarded money put to immediate use.
Dr. Yu purchased a commercial inverted microscope for fluorescence imaging and, through a collaboration with the startup company APEMIS, who specializes in scanning microscopy, began tailoring the microscope to the specific needs of the project. Dr. Yu’s graduate student, Tongtong Lu, also joined the project and managed all lab work, initially focusing on optimizing the use of methylene blue or other contrast agents with respect to cell differentiation and image quality.
With Tongtong Lu’s experiments and conversion of the fluorescence microscope underway, Dr. Yu came across a new technology developed by a professor from UC Davis, Dr. Richard Levenson. Coined MUSE, short for Microscopy with Ultraviolet Surface Excitation, this new technology used ultraviolet light to elucidate tissue surface structures at the microscopic level, and it did so without the need for histologic preparation, reducing the diagnostic price-point in a number of clinical applications. Remaining true to his goal of developing cost-effective solutions, Dr. Yu became interested in finding ways to integrate concepts from this new discovery into his breast tumor margin imaging project, an integration that effectively split the project into multiple lines of inquiry, creating new opportunities for potential funding.
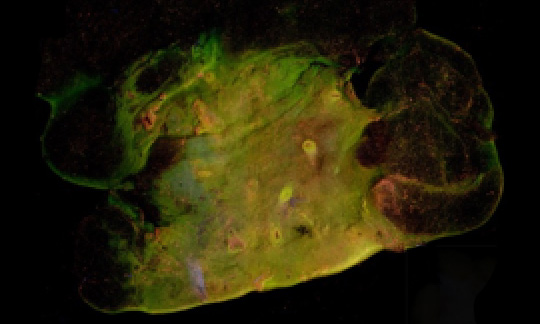 In March of 2018, Dr. Yu’s new breast tumor margin imaging team submitted a Marquette University College of Engineering GHR grant to fund work on the MUSE-based device, which the team named the Deep UV fluorescence scanning microscope, or DUV-FSM. Dr. Yu coordinated with Mollie Patton and Mary Rau from the MCW Tissue bank to acquire discarded breast tissues, and Dr. Julie Jorns, a pathologist from MCW, joined the team, lending her expertise to reading imaged tissue samples, ensuring that the tested medium was correctly distinguishing between cancerous and non-cancerous cells. Finally, in the fall of 2018, Dr. Dong Hye Ye, a deep learning specialist from Marquette University, came on board to facilitate image analysis. With Dr. Ye’s arrival, Dr. Yu’s breast tumor margin imaging team was complete.
In March of 2018, Dr. Yu’s new breast tumor margin imaging team submitted a Marquette University College of Engineering GHR grant to fund work on the MUSE-based device, which the team named the Deep UV fluorescence scanning microscope, or DUV-FSM. Dr. Yu coordinated with Mollie Patton and Mary Rau from the MCW Tissue bank to acquire discarded breast tissues, and Dr. Julie Jorns, a pathologist from MCW, joined the team, lending her expertise to reading imaged tissue samples, ensuring that the tested medium was correctly distinguishing between cancerous and non-cancerous cells. Finally, in the fall of 2018, Dr. Dong Hye Ye, a deep learning specialist from Marquette University, came on board to facilitate image analysis. With Dr. Ye’s arrival, Dr. Yu’s breast tumor margin imaging team was complete.
By early 2019, the initial phases of the project had begun to yield results. The DUV-FSM system was fully ready for tissue imaging. Tongtong had spent several months working with Dr. Gilat-Schmidt to ensure that the use of contrast medium had been optimized for their objectives. The study using discarded human breast tissues from the MCW Tissue Bank had been approved by the MCW IRB, and the first ten fresh surgical samples imaged using the team’s developing technology showed excellent visual contrast in color, tissue texture, cell density and shape between invasive carcinomas and their normal counterparts.
By early 2020, over 50 breast tissue samples had been imaged by the DUV-FSM, and corresponding Hematoxylin and Eosin (H&E) images had been collected by Dr. Jorns. The images had been visually evaluated by a group of non-medical researchers, with accurate distinction between cancerous and non-cancerous cells at a sensitivity and specificity of 96 and 92%, respectively. Objective diagnosis using nuclear-cytoplasm ratio (N/C) calculated for each 2 mm x 2 mm patch was able to differentiate patch-level invasive carcinoma from normal breast tissues with reasonable sensitivity (81.5%) and specificity (78.5%), numbers the team were confident would increase even further with the eventual implementation of Dr. Ye’s deep-learning methodology.
In August 2020, the team submitted their work to the Journal of Biomedical Optics, a prominent journal in the field of biomedical optics focusing on technique and developing technologies. The project struck a chord. Review from the journal came back in just over a month. The journal requested further details and some minor clarifications, but the paper had been accepted for publication, with images from the study chosen to grace the publication’s cover, a great honor in the world of scientific paper publishing.
When asked why he believed the project was chosen for the cover, Dr. Yu responded with the following: “Our approach is simple, and it generates wonderful visual contrast.” He went on to add, “The editor of the Journal of Biomedical Optics is an expert on margin assessment. I am sure he is the person who chose our study for the cover page. He said he was very excited about our study, that we have done terrific work, and that the images are stunning.”
With December 2020 fading faithfully into the past, Dr. Yu’s breast tumor margin assessment project continues moving forward. Tongtong Lu and Dr. Julie Jorns continue their work processing samples. Dr. Ye continues his investigation of deep-learning applications, and the team is actively seeking more extensive funding to further improve the capabilities of their technology. And while the thus far ten-year journey of Dr. Yu’s project has not yet reached the point of care, 2020’s hard-earned praise proved a welcome punctuation, offering opportunity for gratitude and reflection. I sat with Dr. Yu as he looked back on how his idea traveled from concept to cover, reflecting on the project’s many successes and disappointments, redirects, and collaborative contributions. While discussing the project, and all the wonderful people he has worked with along the way, Dr. Yu reiterated his commitment to developing medical solutions that positively impact the health of women, particularly those in low- and middle-income settings.
View more BME News

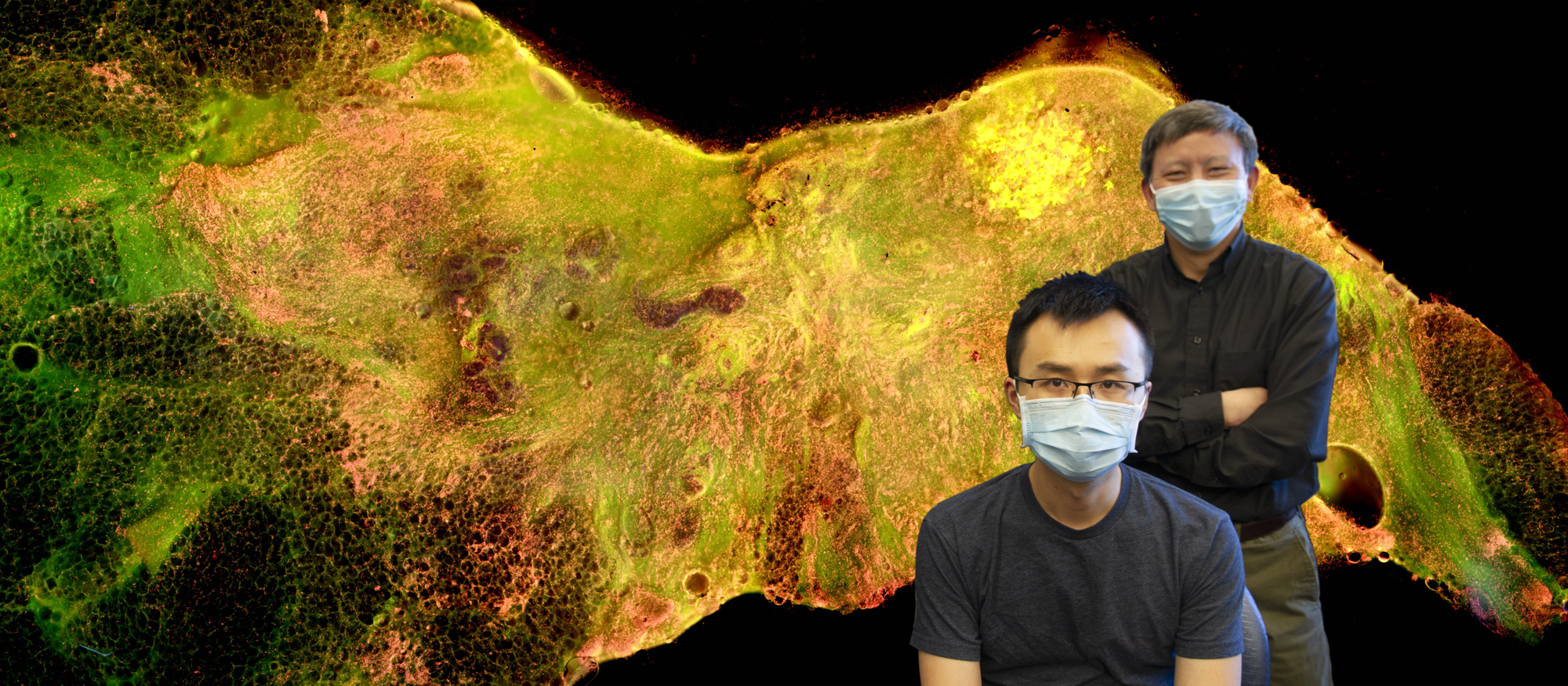
 In March of 2018, Dr. Yu’s new breast tumor margin imaging team submitted a Marquette University College of Engineering GHR grant to fund work on the MUSE-based device, which the team named the Deep UV fluorescence scanning microscope, or DUV-FSM. Dr. Yu coordinated with Mollie Patton and Mary Rau from the MCW Tissue bank to acquire discarded breast tissues, and Dr. Julie Jorns, a pathologist from MCW, joined the team, lending her expertise to reading imaged tissue samples, ensuring that the tested medium was correctly distinguishing between cancerous and non-cancerous cells. Finally, in the fall of 2018, Dr. Dong Hye Ye, a deep learning specialist from Marquette University, came on board to facilitate image analysis. With Dr. Ye’s arrival, Dr. Yu’s breast tumor margin imaging team was complete.
In March of 2018, Dr. Yu’s new breast tumor margin imaging team submitted a Marquette University College of Engineering GHR grant to fund work on the MUSE-based device, which the team named the Deep UV fluorescence scanning microscope, or DUV-FSM. Dr. Yu coordinated with Mollie Patton and Mary Rau from the MCW Tissue bank to acquire discarded breast tissues, and Dr. Julie Jorns, a pathologist from MCW, joined the team, lending her expertise to reading imaged tissue samples, ensuring that the tested medium was correctly distinguishing between cancerous and non-cancerous cells. Finally, in the fall of 2018, Dr. Dong Hye Ye, a deep learning specialist from Marquette University, came on board to facilitate image analysis. With Dr. Ye’s arrival, Dr. Yu’s breast tumor margin imaging team was complete. 