Targeted therapy for Middle Ear Infections
Dr. Joshi's newly patented nanoparticle seeks to revolutionize pediatric ear care
Middle ear infection is the most common cause of doctor visits for children. As a result of the tight passageway created by the size of the young eustachian tube, in conjunction with the tube’s only slightly downward slope in children, secretions and fluid buildup normally draining from the middle ear and into the sinuses often becomes trapped, creating an enticing environment for proliferation of infectious agents such as bacteria. As we move into adulthood, the diameter of the tube widens and the slope becomes more severe, which is why the problem of the middle ear infection seems to all but disappear, but before this happens, it is estimated that the average child will have been prescribed up to 17 full courses of antibiotics meant to help treat this common childhood malady.
The middle ear infection is notoriously difficult to treat. The anatomy of the middle ear is especially designed to provide excellent physiological barriers to damage and disease, and this anatomy has long prevented the effective treatment of middle ear infection. Surrounded in most respects by bone, three small openings to the tightly enclosed chamber are sealed with a triple layer of cells and tissue matrices that prove difficult to permeate. This includes the oval and round windows, which lead to the auditory nerves and Eustachian tube, respectively, and the tympanic membrane, also known as the eardrum. In addition, the bony architecture encasing the ear is complemented by a special barrier, known as the blood-labyrinth barrier, that prevents the passage of blood and other fluids into and out of the inner ear, making medication taken by mouth largely ineffective. In fact, some studies have shown the therapeutic agents taken by mouth never reach the inner ear, though their presence in the body will still contribute to unpleasant gastric side-effects and eventual ineffectiveness of the drug prescribed, particularly in the case of antibiotics. Despite this, oral antibiotics, while not administered immediately upon diagnosis, remain the malady’s first avenue of clinical treatment.
Beyond the chronic and seemingly inescapable burden of avoiding baths and swimming pools, an inability to effectively treat middle ear infections can have serious consequences. Recurrent or severe cases of middle ear infection can cause deafness, as well as traumatic infections in the cavities surrounding the inner ear, which may impact speech and cognitive development. When infections are either chronic or severe, and antibiotics have proven ineffective, surgical implantation of tympanostomy tubes may be required. Though not an ideal solution, tympanostomy tubes puncture the eardrum, creating an artificial avenue through which fluids accumulating in the middle ear can be drained and medications designed to treat infection of the middle ear may be administered. All of this could be avoided, of course, if a reliable, localized drug-delivery system were capable of diffusing through the eardrum and targeting the infection directly.
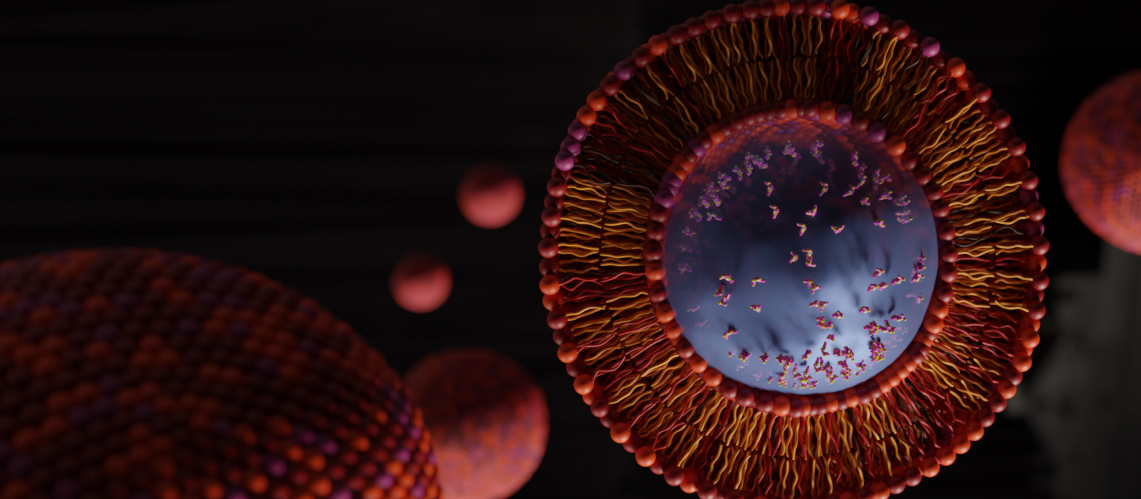
One rapidly evolving potential solution to the problem of effectively treating middle ear infection comes from the field of nanotechnology, and more specifically, the engineering of bilayer, lipid-based nanoparticles capable of carrying anti-infectious agents, such as antibiotics, and specifically designed to diffuse through the body’s outer barrier to the middle ear, the eardrum. Dr. Amit Joshi, Associate Professor and Vice Chair of Research and Clinical Affairs for the Marquette University and Medical College of Wisconsin Joint Department of Biomedical Engineering, is just one researcher working to solve this problem, and with a recent patent of a cationic liposomal nanoparticle, it seems a potential solution might be just over the horizon.
Working in collaboration with Dr. Joseph Kerschner, a pediatric otolaryngologist at the Medical College of Wisconsin, Dr. Joshi’s nanoparticles are engineered to be hydrophobic on the outside, allowing them to remain intact for a period of time before disintegrating, and hydrophilic on the inside, allowing them to effectively trap an aqueous suspension loaded with a given therapeutic agent, such as antibiotics. In addition, the net surface charge of a carrier can be manipulated such that, when coming into contact with the dense, tri-layer epithelial mesh of the eardrum, which normally repels water and other substances used to carry medicine, these nano-carriers are able to passively diffuse and enter into the middle ear intact. After entering the middle ear, the lipid carriers dissolve, releasing the therapeutic agent directly to the source of the infection, whether viral or bacterial.
I caught up with Dr. Joshi since the acceptance of his new patent to glean further details about the nature of his liposome, as well as next steps in the inevitable race to market. According to Dr. Joshi, the distinction of his new patent is the discrete composition of the antibiotics-loaded lipid nanoparticle, which is otherwise currently unimitated in the field, and the manipulability of the net surface charge of the lipid carrier is key to its success. In testing, both steroidal and antibiotic cargos were successfully delivered by the to the middle ear, making the liposomal nanoparticle superior to other, currently available local drug-delivery methodologies, such as otic drops and gels. In addition, delivered therapies retained their therapeutic efficacy, which is to say, they still killed the bacteria.
Despite the recent excitement granted by the patent, Dr. Joshi admits that there is still much work to be done, and his laboratory is gearing up for the next round of testing. “We still need to look at diffusion of the therapeutic agent from the middle ear to the inner ear, for example,” and his lab will work to determine how long the therapeutic agent remains in the middle ear once administered. He notes, additionally, that movement on these types of projects is currently slow, which is a result of supply chain issues and availability of necessary supplies. Nevertheless, he remains excited about this new advancement, as well as the potential for eliminating the need for pediatric tympanostomy tube placement, as well as the overuse of systemic antibiotics in attempts to treat middle ear infection in children.
About Dr. Amit Joshi
 Dr. Amit Joshi is an Associate Professor and Vice Chair of Research for the Marquette University and Medical College of Wisconsin Joint Department of Biomedical Engineering, as well as founding director of the Nanomedicine and Image-Guided Interventions Laboratory located in the Translational and Biomedical Research Center at the Medical College of Wisconsin, Milwaukee Campus. Dr. Joshi's research seeks to develop minimally invasive and non-toxic diagnostic and therapeutic technologies directed at cancer, pulmonary and infectious disease, and other vascular pathologies using nanotechnology and novel imaging techniques.
Dr. Amit Joshi is an Associate Professor and Vice Chair of Research for the Marquette University and Medical College of Wisconsin Joint Department of Biomedical Engineering, as well as founding director of the Nanomedicine and Image-Guided Interventions Laboratory located in the Translational and Biomedical Research Center at the Medical College of Wisconsin, Milwaukee Campus. Dr. Joshi's research seeks to develop minimally invasive and non-toxic diagnostic and therapeutic technologies directed at cancer, pulmonary and infectious disease, and other vascular pathologies using nanotechnology and novel imaging techniques.
Learn more about Dr. Joshi
Learn more about NIGIL

 Dr. Amit Joshi is an Associate Professor and Vice Chair of Research for the Marquette University and Medical College of Wisconsin Joint Department of Biomedical Engineering, as well as founding director of the Nanomedicine and Image-Guided Interventions Laboratory located in the Translational and Biomedical Research Center at the Medical College of Wisconsin, Milwaukee Campus. Dr. Joshi's research seeks to develop minimally invasive and non-toxic diagnostic and therapeutic technologies directed at cancer, pulmonary and infectious disease, and other vascular pathologies using nanotechnology and novel imaging techniques.
Dr. Amit Joshi is an Associate Professor and Vice Chair of Research for the Marquette University and Medical College of Wisconsin Joint Department of Biomedical Engineering, as well as founding director of the Nanomedicine and Image-Guided Interventions Laboratory located in the Translational and Biomedical Research Center at the Medical College of Wisconsin, Milwaukee Campus. Dr. Joshi's research seeks to develop minimally invasive and non-toxic diagnostic and therapeutic technologies directed at cancer, pulmonary and infectious disease, and other vascular pathologies using nanotechnology and novel imaging techniques. 